Deck Review with Gemini Therapeutics
Surveying great inventors and businesses
More well thought out work can be found at — https://axial.substack.com/
Axial partners with great founders and inventors. We invest in early-stage life sciences companies often when they are no more than an idea. We are fanatical about helping the rare inventor who is compelled to build their own enduring business. If you or someone you know has a great idea or company in life sciences, Axial would be excited to get to know you and possibly invest in your vision and company . We are excited to be in business with you — email us at info@axialvc.com
Gemini Therapeutics was founded in 2015 to bring precision medicine to age-related macular degeneration (AMD), other ocular diseases, and systemic diseases driven by complement. The company uses a wide set of technologies from monoclonal antibodies and other biologics to AAV gene therapies. Its platform has generated the first recombinant Complement Factor H, which regulates complement activation, to focus first on dry AMD. Gemini’s business model is unique because if patients respond to a biologic, the plan is to switch them over to an AAV gene therapy.
The first slide of their latest corporate presentation shows Gemini’s focus on precision medicine in AMD.
The next slide goes into Gemini’s highlights: a completed phase 1 study of their lead asset, a recombinant Complement Factor H (CFH), and ongoing phase 2 studies in advanced Dry AMD with CFH variants.
The company lays out their pipeline of biologics and AAVs in AMD along with an mAb in renal disease.
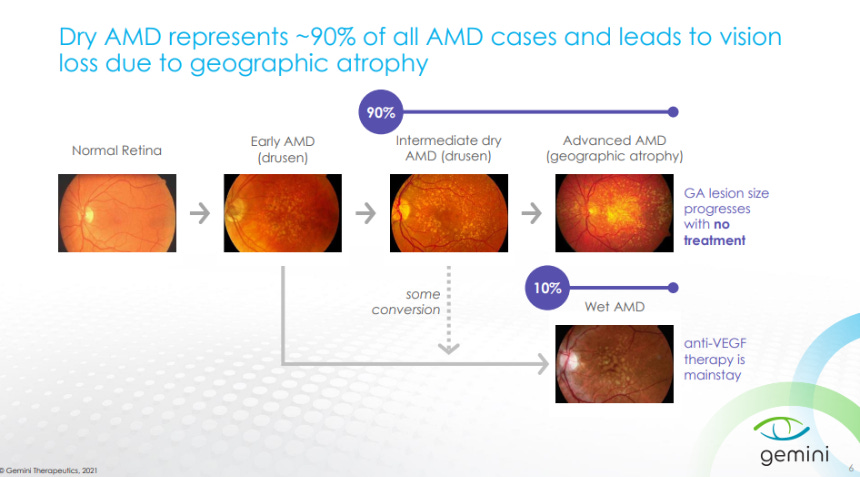
An overview on Dry AMD is given where the majority of patients progress to vision loss with no currently approved treatments and the rest progress to wet AMD that can be treated with an anti-VEGF antibody treatment.
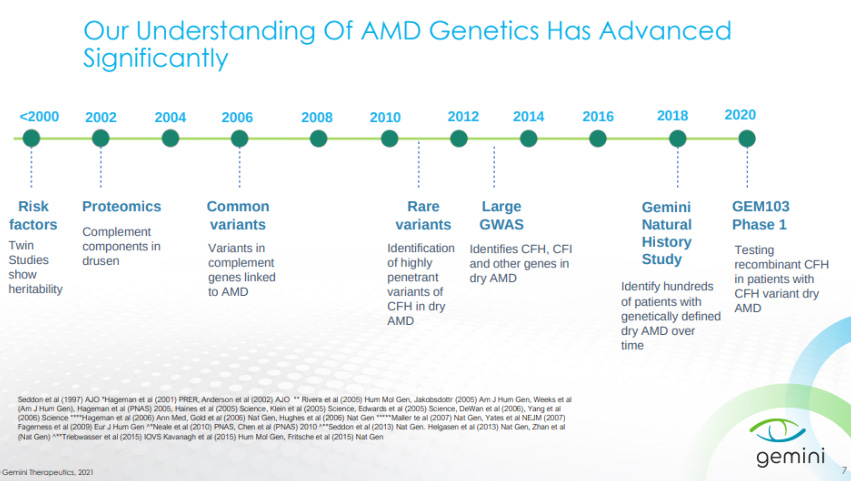
Over the last 2 decades, AMD genetics has been characterized with variants in CFH, Complement Factor I (CFI), among others.
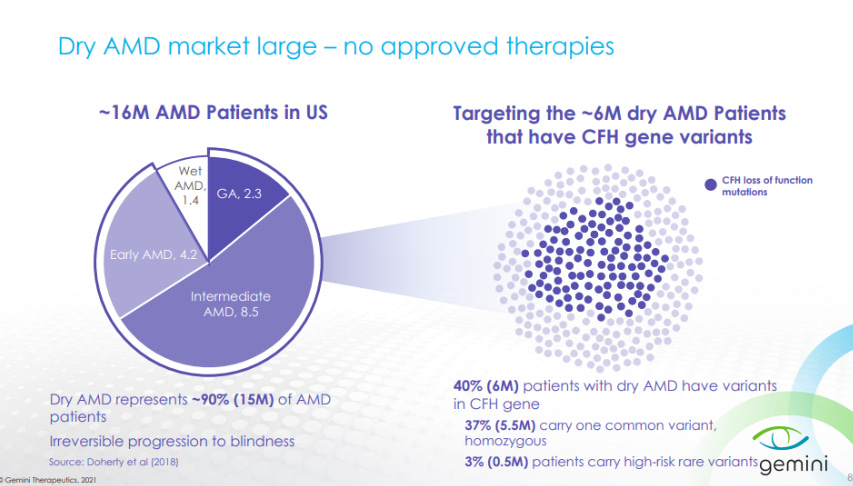
Overall, the Dry AMD market is composed of around 16M patients in the US with 6M of them having genetic variants in the CFH gene.
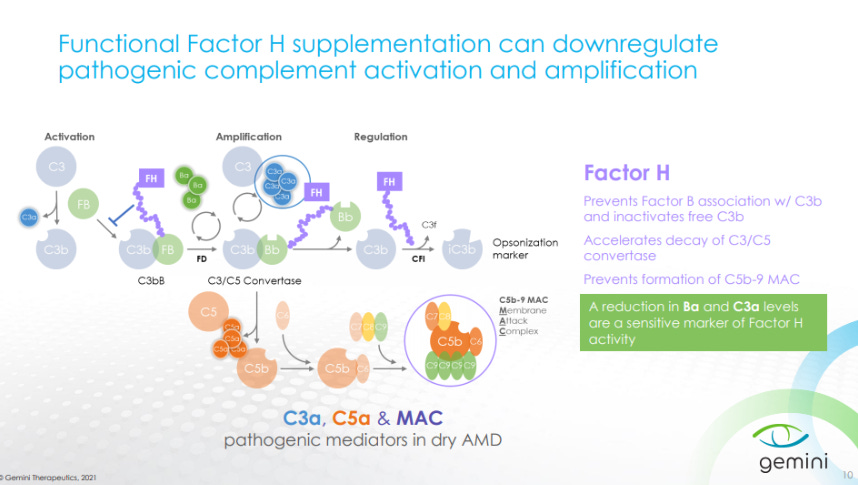
Then Gemini given an overview on Complement Factor H along with its role in Dry AMD.
CFH can be used to reduce complement activity and treat symptoms of Dry AMD.
Which is why Gemini’s lead drug candidate is a recombinant CFH. It’s not clear why they were the first to do this though? Maybe the genetics had to be figured out where it was worth investing resources to manufacture a CFH.
The next slides go into clinical data for the lead drug candidate, GEM103. Inhibiting components of complement (C3) with a monoclonal antibody leads to toxicities such as hemolysis and reductions in phagocytosis for clearance. Phase 1 data established GEM103’s ability to inhibit complement activity in patients with lower levels of these toxicities.
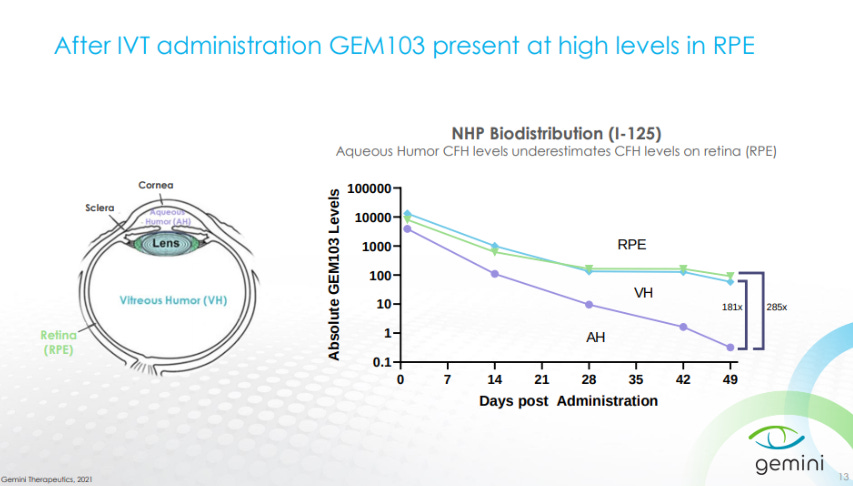
GEM103 showed an ability to remain in the retina a month after treatment after intravitreal (IVT) injection.
Phase 1 data for GEM103 showed safety and tolerability without any dose-limiting toxicities (DLT).
Across all 4 doses, GEM103 showed an ability to increase CFH above baseline after 1-2 months.
Factor Ba is a fragment of Complement Factor B and was used as a biomarker for complement activity - GEM103 was found to reduce Ba 2 months post-treatment.
Which occurred across most doses of GEM103.
The following slide describes the safety data on Gemini’s lead drug candidate.
The next goes into the phase 2 trial design to focus on patients with CFH loss-of-function.
And do a dose escalation study over 6 months.
The second to last slide goes into the various milestones for Gemini: phase 2 studies for GEM103 in Dry AMD along with getting their AAV drug candidate into the clinic by ~2022.
Gemini ends the presentation with the highlights slide again.
Gemini’s presentation does a good job at showing their progress in developing a pipeline of CFH therapeutics for Dry AMD. The company is building a unique business and the presentation could do an even better job at conveying why pursuing CFH variants in Dry AMD is important given the high unmet clinical need.
Follow up questions for the team:
Why were the first to bring a recombinant CFH to the clinic?
If both the recombinant CFH and AAV therapies are approved, how does Gemini think about timing of use of either?
What kind of platform technologies have been built out on the genomics side? Any updates on other retinal diseases or complement-driven ones?